FEATURE
Small Molecule, Big Discovery
Hydrofracking has created an ecological problem. Stephen Tajc, Ph.D., may have the solution.
by Erich Van Dussen

It can be called hydraulic fracturing, hydrofracking, or simply fracking. But by any name, the energy-gathering process is a controversial topic that has drawn passionate debates over its safety and usefulness.
To Stephen Tajc, Ph.D., however, fracking is not so much a controversy as a scientific puzzle involving one small molecule and billions of gallons of water. The solution to the puzzle—following many, many more lab hours still to come—could resolve a major ecological problem associated with this contentious modern issue.
As is the case with many scientific breakthroughs, this one was revealed through a convergence of earlier seemingly unrelated studies. A few years back, two separate research projects were being conducted by Nazareth chemistry majors. One explored the effects of a bacteria that used the small molecule, dipicolinic acid (DPA), as a source of nutrients when calcium is present; when the DPA was added to water that contained calcium, most of that metal would fall out of the water as a precipitant.
“DPA is a well-researched molecule that [scientists] don’t have a lot of interest in anymore,” says Tajc, assistant professor of organic chemistry. “I think it’s considered kind of boring.”
The second research project was devoted to hydrofracking, and when Tajc heard of the two parallel studies, a light bulb went off. In fracking, the presence of metal in water is a big problem. Could that small molecule be the solution?
Water, Water Everywhere…
First employed in the mid-20th century, hydraulic fracturing is today the most common technique for obtaining natural gas from shale rock beds buried deep beneath our planet’s surface. The process requires drilling thousands of feet, then injecting a high-pressure solution that eventually cracks the rock, creating fissures from which the gas can escape and be captured.
A typical fracking liquid is comprised of fresh water—millions of gallons are used in every fracking well—mixed with chemical additives that amplify its effectiveness in the subterranean process. But after spending time underground amid all those minerals, the water is anything but fresh: It becomes suffused with extremely high levels of calcium, barium, magnesium, strontium, and other metals that are toxic in high doses. (Strontium, for example, has radioactive properties that have no place in potable water.)
According to Environmental Protection Agency guidelines, fracking wastewater contains 400,000 times the maximum allowable safe levels of those minerals—far beyond the capabilities of conventional water purification processes. As it can’t be returned to the water supply or re-used for fracking, the wastewater is often disposed of through injection back into the earth; but as fracking activity has increased throughout the United States, the pent-up pressure created by that underground disposal has been linked to fracking-related earthquakes. An alternative storage method involves holding ponds in which the water is left to evaporate over time—but there are several reports of fracking holding ponds in the southwestern U.S. that have leached contaminated water into the soil and local water supply.
Would it be fair to call fracking water … toxic waste? “Well, it’s definitely waste, and it’s definitely toxic,” Tajc says. “Yes, I’d say that’s fair.”
The DPA Solution
Finding a way to remove the minerals from the water would eliminate the need for these risky disposal methods and enable potable water to be returned to our country’s available supply. “When you think about the serious water shortages experienced by the western half of the country, that alone is an issue worth addressing,” Tajc says.
Inspired to combine the principles behind those two studies, he headed to the lab to begin the process of determining DPA’s effectiveness in stripping the harmful metals from fracking wastewater. “We found we could extract 95 percent of the calcium by using this small molecule,” he says. “Then it turned out that the process also worked on strontium. It’s not as efficient [as calcium]—I think we’ve seen as high as 65 to 70 percent removal—but that’s better than anything else that’s been shown so far.”
More study is needed to find ways to increase the amount of calcium and other metals that can be removed from fracking wastewater using DPA. In time, he adds, there’s no reason why the same basic methodology couldn’t also be used to remediate water that has been contaminated through other means—with lead, for instance, or even the halogens found in the waters of the Finger Lakes.
“Right from the start we saw very good results from this,” he says. “But I wasn’t satisfied with good. I wanted to make it better.”
A Team Effort
The work to refine and improve DPA’s effectiveness has been going on now for a little more than three years. “At its most fundamental, it’s a simple chemical reaction,” Tajc says. “You need to chemically modify a little bit at a time to see, Did I improve it? Did I make it worse?”
He compares these ongoing research efforts to the iterative steps that must be taken to refine a new medicine before it can be used. “You don’t cure something with the initial discovery—instead, you improve it to make sure it does the best job it can.”
This painstaking approach, called a structure-activity relationship, is critical to nudge the DPA procedure to its ultimate effectiveness. And it’s a team effort: Tajc estimates that more than 40 students have contributed to the project to date, including some who start as early as their freshman year. “The work that we’re doing is the kind of thing I teach in Organic Chemistry,” he says, “so freshmen get a chance to experience those processes before they take the class as a sophomore.”
Shane Fuentes ’18 agrees. Although he didn’t expect to have this kind of opportunity prior to coming to Nazareth, the biochemistry major was excited to learn he would be able to dive into Tajc’s research as a freshman.
“At a larger school I might have had an opportunity to participate in some basic research by now, but I’d probably just be holding test tubes for a senior or a grad student,” says Fuentes, now a sophomore. “And it made Organic Chemistry a lot easier when I took the course.”
The practice of bringing students into research projects early is becoming more commonplace at Nazareth, says William Lammela, Ph.D., chair of the Chemistry and Biochemistry Department. “The research makes the knowledge real. It brings the content alive,” he says. “And in this case they can relate to it in an even more powerful way—they can see how work they’re participating in is directly addressing a social problem.”
Tajc is also supported in his DPA research by his fellow faculty: he’s working with Lammela on the analytical chemistry that helps determine exactly how much of the metals have been removed from the water; and Stephanie Zamule, Ph.D., assistant professor of biology, is assisting on the portions of his work that delve into toxicology and microbiology. “Those aspects are out of my area of expertise,” Tajc says. “It’s wonderful to work in an environment where this kind of collaboration can occur.”
Teachable Moments
Tajc is uncertain how long it will be before his process is ready for commercial applications, but 2016 could be a big year for the DPA technique: In January he presented his research to date as a keynote speaker at the international Latin American Congress of Chemistry in Concepcion, Chile. “I’ve never given away what the bacteria is or exactly how we do the process before—this [was] the first time,” he says.
The reaction to Tajc’s work was uniformly positive, though muted by South America’s lack of involvement in hydraulic fracturing. Still, experts at the Chilean conference quickly picked up on the broader applicability of his research. “They began to see how the process could improve water quality across the board,” he says. “Copper mining is a major industry in that country, for example, and this process might be able to play a role in that.”
Chile was his first detailed presentation on this topic, but three years ago Tajc presented the fundamentals of what would become his DPA technique at an American Chemical Society Nation Meeting in San Francisco that featured a hydraulic fracturing symposium. There, he discovered his was a lone voice advocating for innovative solutions to the issue. “Everyone was talking about the effects of the contaminated wastewater, but no one was talking about ways to remediate it,” he says.
Following his San Francisco presentation, a representative of a major oil company approached Tajc to praise his early work. “He called it a ‘game-changer’ for hydrofracking,” Tajc recalls. “That was exciting to hear … it helped show me that we were on to something.”
“A Neutral View”
Although the potential benefits of Tajc’s research would resolve some of the negative aspects of hydraulic fracturing, the educator says he’s not taking a stand on the issue of fracking itself.
“I try to have a very neutral view,” he says. “No matter what type of process you’re getting energy from, there’s always going to be some sort of environmental impact. The wastewater is just the effect of this process.”
On the other hand, he’s also a native of southwestern Pennsylvania, where coal mining was once a major source of employment—and where his grandfather, a miner, died of black lung disease. Now, hydraulic fracturing in that region’s Marcellus Shale formation has bolstered a once-foundering economy.
“Hydrofracking has brought life back into my hometown, but at what cost?” Tajc wonders. “On the surface it’s not nearly as bad [as coal mining], but it’s only been going on for 10 years. We don’t really know yet.
“That’s why I’m trying to take the middle road. I see a recognized problem, and I’m trying to offer a solution.”
With the first detailed presentation of his work behind him, Tajc is now working on a scholarly article that he hopes to see published this year. It may be years before the full industrial potential of the DPA process can be realized, but he’s excited that his research, even in these early stages, might be able to “spark a conversation” on the issue of what to do about fracking wastewater.
And in the meantime, as a scientist he’s simply looking forward to getting as much feedback as possible. “When it comes to science, the peer review is everything,” he says. “You may think you’re doing phenomenal work, but if others don’t agree, you can learn from that as well.”
Erich Van Dussen is a freelance writer in Rochester, New York.
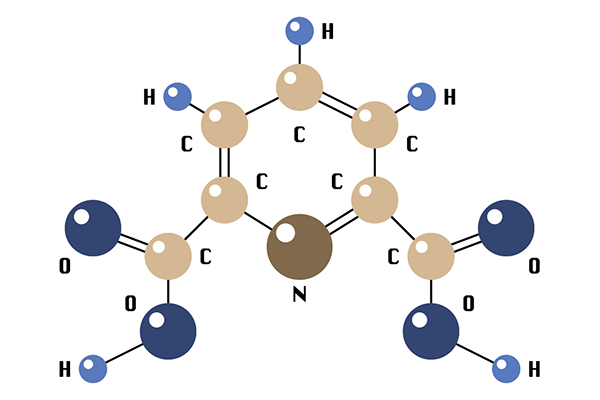
dipicolinic acid (DPA)
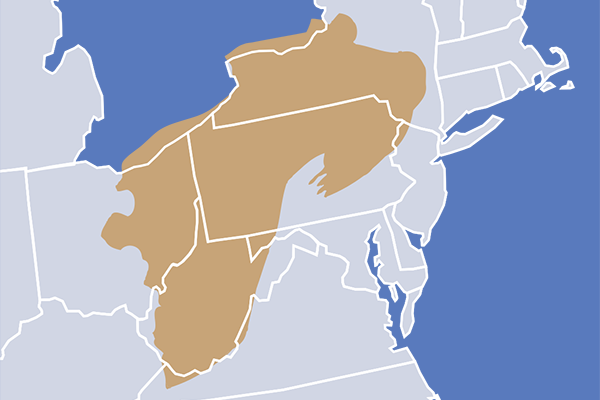
Marcellus Shale formation